A Metal That Forms an Oxide of Formula R2o3

Solved Choose The Substance Which Fits The Set Of Chegg Com


Chapter 8 Electron Configuration And Periodicity 1

Chapter 8 Electron Configurations And Periodicity Contents And

A Metal Oxide Has The Formula Z2o3 It Can Be Reduced By Hydrogen To Give Free Metal And Water 0 1596 G Of The Metal Oxide Requires 6 Mg Of Hydrogen For

Lesson 7 8 Groups Of The Periodic Table

Cerium Uses Properties Facts Britannica

Chapter 8 Electron Configurations And Periodicity Contents And

Unit 7 Quantum Theory Electron Configuration And Periodicity Ppt Download

Transition Metals Often Form Coloured Compounds Transition Metal Metal Ion Chemistry
Solved Use The Building Up Principle To Obtain The Electron Chegg Com
Unit 7 Quantum Theory Electron Configuration And Periodicity Ppt Download

Unit 7 Quantum Theory Electron Configuration And Periodicity Ppt Download

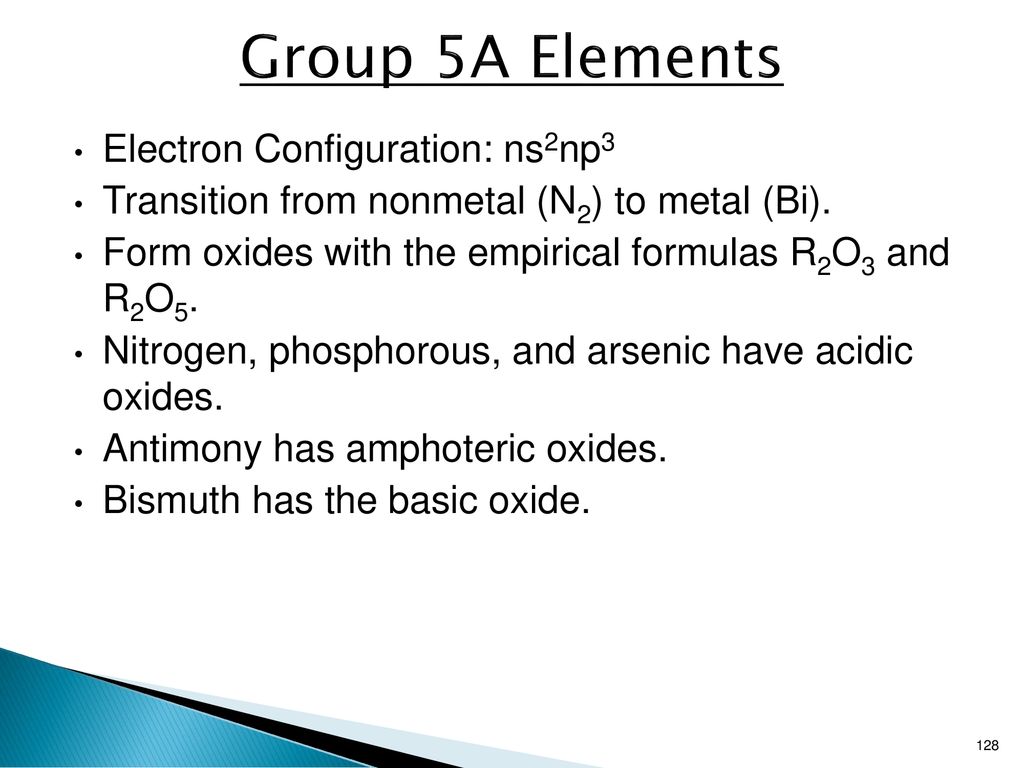
An Element R Forms The Highest Oxide R2o5 R Belongs To

Chapter 8 Electron Configurations And Periodicity Contents And

An Element R Forms The Highest Oxide R2o5 R Belongs To

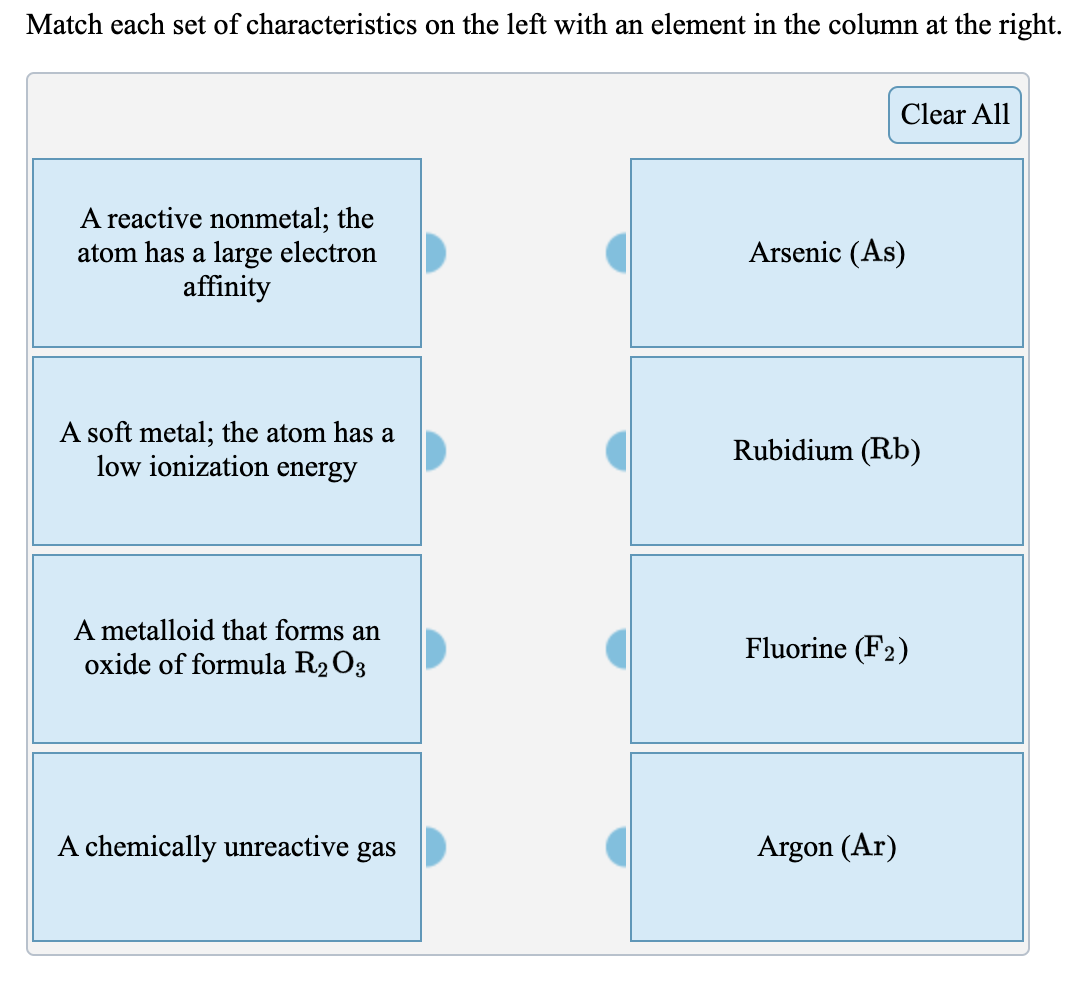
Solved A A Reactive Pale Yellow Gas The Atom Has A Large Chegg Com

Unit 7 Quantum Theory Electron Configuration And Periodicity Ppt Download


Comments
Post a Comment